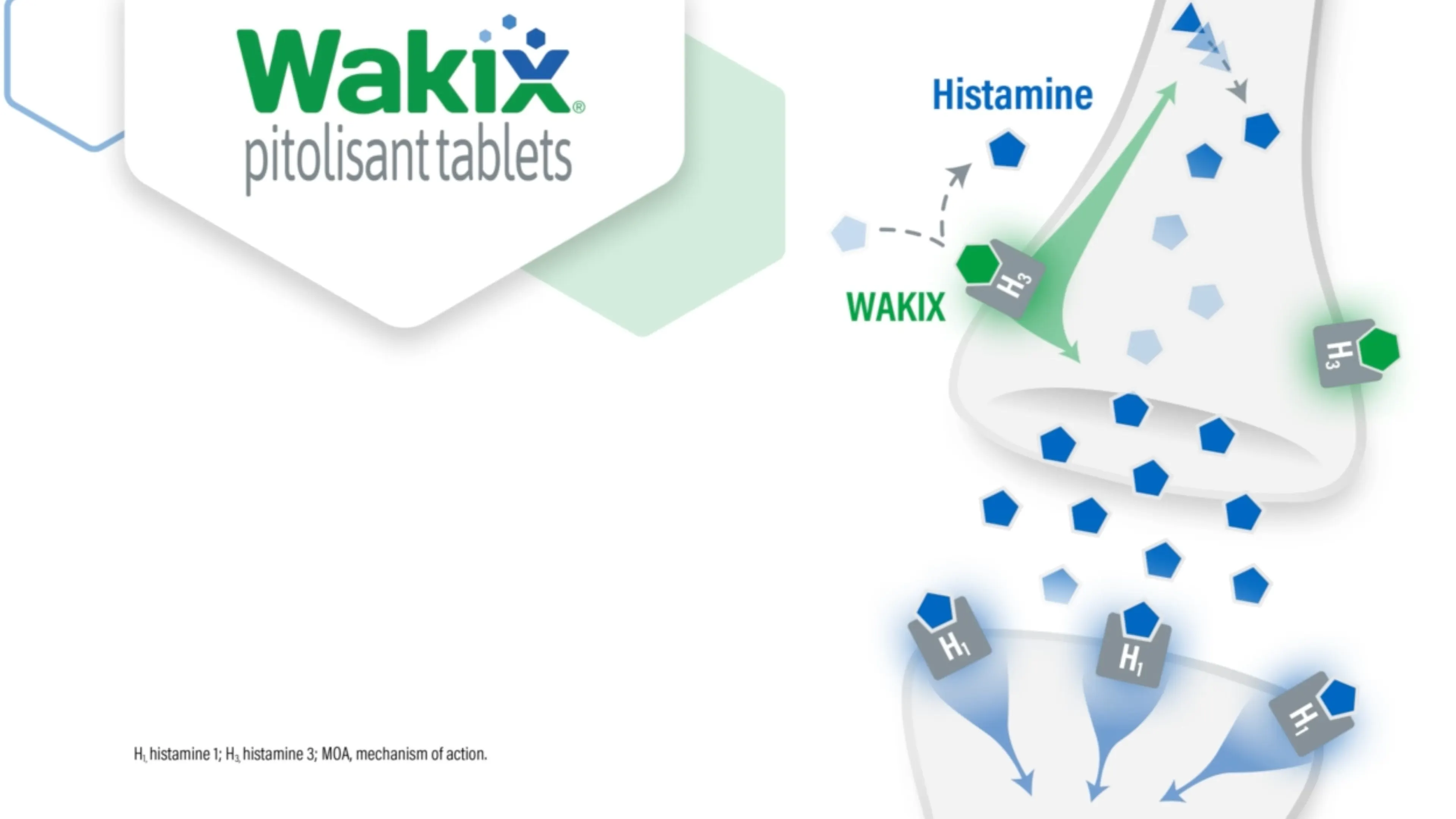
WAKIX Increases Histamine Levels in the Brain
The mechanism of action (MOA) of WAKIX in EDS in patients 6 years and older with narcolepsy or cataplexy in adult patients with narcolepsy is unclear; however, its efficacy could be mediated through its activity as an antagonist/inverse agonist at H3 receptors, which results in increased histamine levels in the brain.